Chemical and Materials Engineering
NANO-STRUCTURED PARTICLES FOR IN-SITU TCE DESTRUCTION
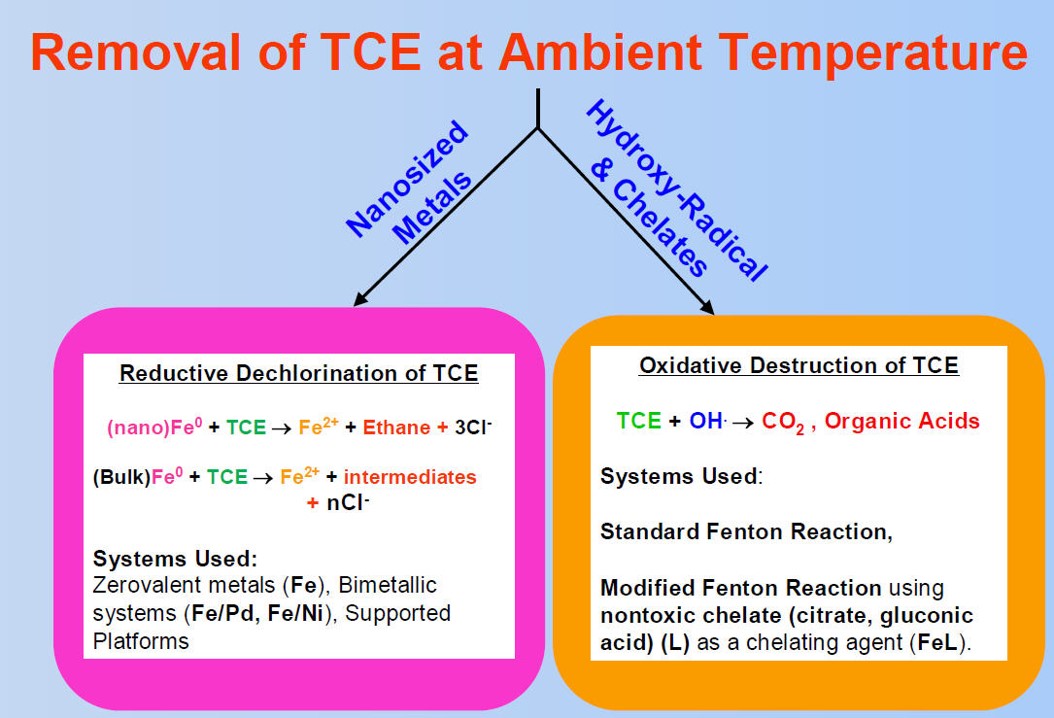
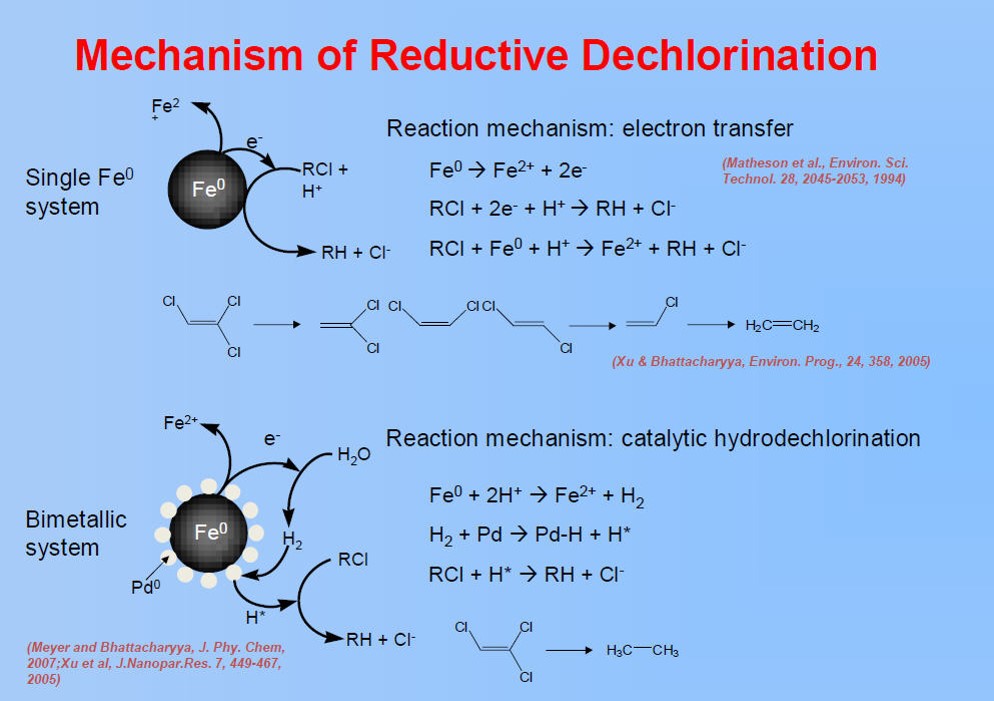
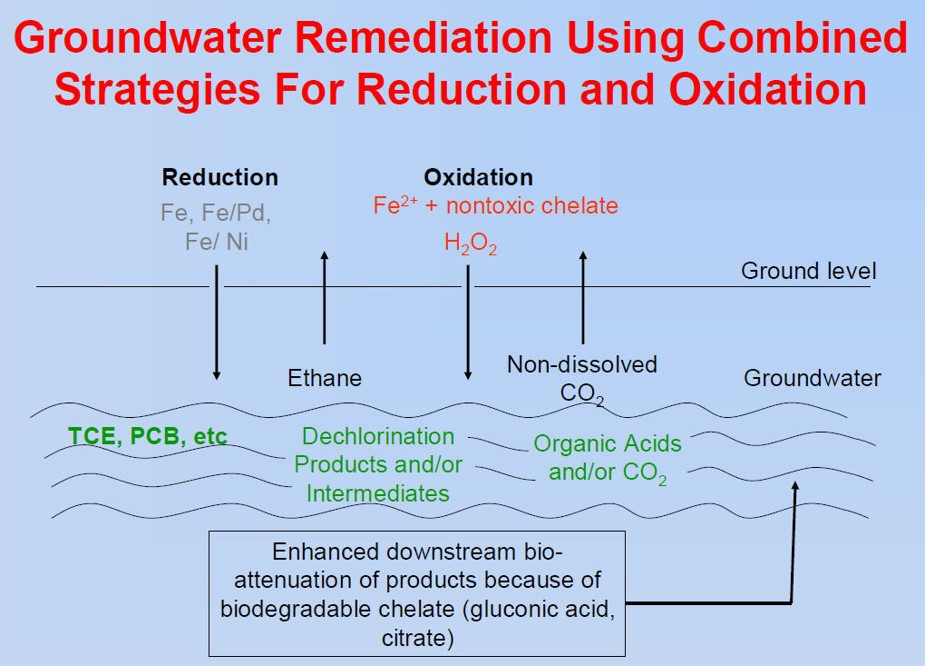
A project team of UK and UC Berkeley faculty, post-doctoral students, graduate students and researchers completed bench-scale development and evaluation of oxidative TCE destruction utilizing a chelate-modified Fenton reaction and reductive destruction using bi-metallic (Fe/Ni and Fe/Pd) nanoparticles to dechlorinate trichloroethylene (TCE) in situ.
The Project was jointly supported by KRCEE and the UK NIEH Superfund Basic Research Program through the Kentucky Water Resources Research Institute.
Project goals included:
► Development of effective methods for the dechlorination of toxic organics at ambient temperatures
► Determine role of dopant metal in bimetallic nanoparticle reactivity
► Study potential for on-site generation of chemicals needed for chelate-modified Fenton reaction
► Determine effectiveness of both reductive and oxidative dechlorination in column studies to simulate groundwater flow
► Development of a Treatability Study Work Plan for preliminary ex-situ and in-situ testing of oxidative and reductive TCE dechlorination at the PGDP.
Column tests utilizing PGDP vicinity Regional Gravel Aquifer groundwater and media were part of the nanoparticle (nanoaggregate) and modified Fenton reaction laboratory testing protocols.
Circulating batch-column experiments were used to study dechlorination under flow conditions and demonstrate the ability of non-stabilized Fe/Pd nanoaggregates to remove significant amounts of TCE (80–90%) over a broad range of groundwater velocities (12.9–83 ft per day) using moderate metal loadings (0.23–0.5 g L−1).
Project experimentation proved the non-toxic chelate modified Fenton reaction effectively dechlorinated TCE in both the aqueous and organic phases at pH 6–7 using low H2O2:Fe(II) molar ratios (4:1 to 8:1).
Detailed information on the Project’s methods and outcomes are summarized in the Project reports, presentations and journal articles below.
Project Participants
Dr. Dibakar Bhattacharyya, Chemical and Materials Engineering, University of Kentucky (Co-PI)
Dr. Lindell Ormsbee, PE, Director, Kentucky Water Resources Research Institute (KWRRI); Director, Kentucky Research Consortium for Energy and Environment; Professor, Department of Civil Engineering, University of Kentucky (Co-PI)
Dr. Rodney Andrews, PE, Director, Center for Applied Energy Research; Director, Kentucky Research Consortium for Energy and Environment, University of Kentucky
Dr. David Sedlak, University of California Berkeley
Dr. David Meyer, Postdoctoral Researcher, Department of Chemical and Materials Engineering, University of Kentucky
Dr. Vasilie Smuleac, Chemical and Materials Engineering, University of Kentucky
Dr. Leonidas Bachas, Associate Dean, College of Arts & Sciences, Department of Chemistry, University of Kentucky
Scott Lewis, Doctoral Student, Department of Chemical and Materials Engineering, University of Kentucky
Noah Meeks, Chemical and Materials Engineering, University of Kentucky
Andrew Lynch, Undergraduate Student, Chemical and Materials Engineering, University of Kentucky
James Kipp, Associate Director, Kentucky Water Resources Research Institute (KWRRI), University of Kentucky
Dr. Y. Li, Postdoctoral Researcher, Department of Chemical and Materials Engineering, University of Kentucky
Dr. J. Xu, Postdoctoral Researcher, Chemical and Materials Engineering, University of Kentucky
Y. Tee, Doctoral Student, Chemical and Materials Engineering, University of Kentucky
Steve Hampson, Associate Director, Kentucky Research Consortium for Energy and Environment (KRCEE), Center for Applied Energy Research, University of Kentucky
Project Documents
TCE BIOACCUMULATION BY MICROALGAE
TCE is a common xenobiotic contaminant that is recalcitrant to degradation. Widespread and prolonged use of this volatile solvent in industrial applications has led to extensive contamination of soils, groundwater, and surface water.
In situ remediation technologies, such as natural attenuation, phytoremediation, and biodegradation are typically used for the removal of TCE from contaminated sites. However, these techniques may require several decades to achieve acceptable treatment goals. Thus, there is a need to develop more efficient, cost-effective, and low maintenance methods for remediation (O’Neill et al, 1999).
Ponds contaminated with TCE have been known to support the growth of microalgae. The utilization of microalgae to take up TCE from the ponds, also referred to as bio-concentration, may be an effective path for TCE removal from the ponds. In order for microalgae to be used for TCE removal, the microalgae must be able to grow in the presence of TCE, which was shown by Biggs and coworkers (1979). Second, the microalgae must be able to bio-accumulate TCE, which was shown to be the case with Chlorella vulgaris and Scenedesmus quadricauda (Smets and Rittman, 1990). Third, the system needs to be tested in a laboratory setting to determine the effectiveness and possible optimization of the process, which is the focus of this scope of work.
Project work included three tasks:
- Literature review of the previous work with TCE accumulation in microalgae and of the previous work on bio-accumulation of 99Tc.
- Flask experiments (300-mL working volume) to determine rate of uptake, effects of TCE on algae growth, and to gather TCE-rich algae cells to determine subsequent chlorine cleanup needs. Flask experiments focused on the two strains identified previously as TCE bio-accumulators.
- Employment of the bio fence to do a larger scale TCE scrubbing demonstration.
Project Participants
Dr. Czarena Crofcheck, PE, Director of Undergraduate Studies, Bio Systems and Agricultural Engineering, University of Kentucky (PI)
Dr. Rodney Andrews, PE, Director, Center for Applied Energy Research; Director, Kentucky Research Consortium for Energy and Environment, University of Kentucky
Dr. Mark Crocker, UK-Center for Applied Energy Research
Steve Hampson, Associate Director, Kentucky Research Consortium for Energy and Environment (KRCEE), Center for Applied Energy Research, University of Kentucky
Project Documents
DISPOSITION OF PADUCAH GASEOUS DIFFUSION PLANT NICKEL
University of Kentucky Paducah Extended Campus Engineering Program faculty completed research into the release, sale and reuse of nickel from the Paducah Gaseous Diffusion Plant. There are an estimated 9,000 tons of radioactively contaminated nickel in the form of ingots stored at the current and former uranium enrichment plants in Paducah, Kentucky, Portsmouth, Ohio and Oak Ridge, Tennessee.
Nickel has a particularly high scrap value, but a lack of U.S. standard for release of volumetrically contaminated material and a moratorium by the DOE on commercial reuse of any radioactively contaminated metals prevents sale or reuse of volumetrically contaminated material – even within the nuclear complex.
Project research identified the regulatory framework and roadblocks which impact release of volumetrically contaminated material. Project recommendations include the establishment and validation of a process whereby the contaminated nickel can be made as clean as or cleaner than conventional commercially available nickel.
Project Participants
Dr. David Silverstein, PE, Assistant Professor, Paducah Extended Campus Program, Chemical and Materials Engineering, University of Kentucky (Co-PI)
Dr. Lindell Ormsbee, PE, Director, Kentucky Water Resources Research Institute (KWRRI); Director, Kentucky Research Consortium for Energy and Environment; Professor, Department of Civil Engineering, University of Kentucky (Co-PI)
Project Documents
NICKEL RELEASE SUPPORT
University of Kentucky Paducah Extended Campus Engineering Program faculty provided support to the Paducah Area Community Reuse Organization (PACRO) for the organizations efforts to initiate a cleanup and sale of nickel stockpiled at the Paducah plant.
A preliminary recommendation to utilize a modified chemical vapor deposition (CVD) process to was provided to PACRO.
Project Participants
Dr. Jim Smart, Assistant Professor, Paducah Engineering Program, University of Kentucky
Dr. Fuqian Yang, Chemical and Materials Engineering, University of Kentucky
Project Documents
| Project Report | Volpe, J.A., et.al., Market Available Virgin Nickel Analysis Data Summary, KRCEE 9.1 |
| Project Briefing Presentation | PACRO Commercial Nickel Sampling and Analysis Plan (Revision 1) KRCEE 9.2 2003 |
URANIUM REUSE – BATTERY DEVELOPMENT
University of Kentucky Paducah Campus Engineering Program faculty and a project team of industry subject matter experts completed research into the potential use of PGDP’s depleted uranium as battery material in lithium-ion batteries. There are currently more than 5 billion pounds of depleted uranium hexafluoride stored at the PGDP.
Project Participants
Dr. Paul Dunbar, Assistant Professor, Paducah Campus, Chemical and Materials Engineering, University of Kentucky (Co-PI)
Dr. Rhonda Lee-Desautels, Assistant Professor, Chemical and Materials Engineering, University of Kentucky, Paducah Campus (Co-PI)
Dr. Lindell Ormsbee, PE, Director, Kentucky Water Resources Research Institute (KWRRI); Director, Kentucky Research Consortium for Energy and Environment; Professor, Department of Civil Engineering, University of Kentucky (Co-PI)
Walter Tracinski, Applied Power Inc.
Dr. Stephen Lipka, Center for Applied Energy Research, University of Kentucky
Dr. Richard Howard, Boeing Company
Dr. Chris Johnson, Boeing Company
Project Documents
| Project Report | Dunbar, P.D., DeSautels, R.L., Uranium Battery Development Project Final Report, KRCEE p2.1, 2007 |
| Project Status Presentation | Dunbar, P.D., Uranium Battery Project Introduction, KRCEE Quarterly Meeting Presentation, April 2004. |
| Project Status Presentation | Dunbar, P.D., Uranium Battery Project Update, KRCEE Quarterly Meeting Presentation, February 2005. |
| Project Status Presentation | Dunbar, P.D., Uranium Battery Project Update, KRCEE Quarterly Meeting Presentation, April 2006. |
| Project Status Presentation | Dunbar, P.D., Uranium Battery Project Update, KRCEE Quarterly Meeting Presentation, September 2006. |
SEPARATION OF RADIONUCLIDES FROM PGDP NICKEL
The decontamination and radiation decommissioning of the gaseous diffusion process at the PGDP has generated and will generate vast quantities of nickel and other metals volumetrically contaminated with detectable levels of radioactive materials. The most frequently identified contaminant in the PGDP nickel is technetium-99 (99Tc).
Available refining technologies were proven incapable of removing 99Tc to less than detectable levels.
A moratorium on the release of any volumetrically contaminated material containing detectable radiation has precluded the release of the PGDP nickel stockpile.
This project’s research identified properties of 99Tc and nickel and pursued development of a bench-scale separation process.
Project Participants
Dr. Eric Grulke, Professor, Associate Dean Research & Graduate Studies, Chemical and Materials Engineering, University of Kentucky (PI)
Dr. Lindell Ormsbee, PE, Director, Kentucky Water Resources Research Institute (KWRRI); Director, Kentucky Research Consortium for Energy and Environment; Professor, Department of Civil Engineering, University of Kentucky (Co-PI)
Dr. Tony Zhai, Assistant Professor, Chemical and Materials Engineering, University of Kentucky
Dr. Louie El-Azzami, Post-Doctoral Researcher, Chemical and Materials Engineering, University of Kentucky
Dr. Burt Lynn, Chemical and Materials Engineering, University of Kentucky
Steve Hampson, Associate Director, Kentucky Research Consortium for Energy and Environment (KRCEE), Center for Applied Energy Research, University of Kentucky
Project Documents
| Project Briefing Presentation | Grulke, E.A., Recovery of purified nickel from material contaminated with 99Tc, KRCEE Quarterly Meeting Presentation, September 2006 |
| Project Briefing Presentation | Grulke, E.A., Nickel-Technetium Separation by Metal Distillation and Vapor Deposition PACRO Presentation, KRCEE p9.4, 2004 |
| Project Research Review and Proposal | Grulke, E.A., Zhai, T., El-Azzami, L., Separation of Nickel from Technetium, UK/KRCEE Doc #: P9.5 2007 |
| Professional Meeting Presentation | Grulke, E.A., Nickel-Technetium Separation by Metal Distillation and Vapor Deposition PACRO Presentation, UK/KRCEE Doc #: P9.4, August 2004 |